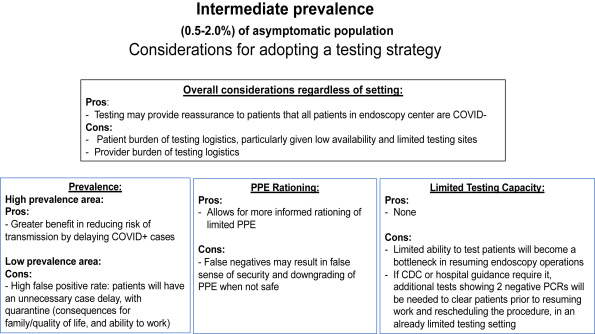
1. For most endoscopy centers where the prevalence of asymptomatic severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is intermediate (0.5%-2%), AGA suggests implementing a pretesting strategy using information about prevalence and test performance (sensitivity/specificity) in combination with considerations about the benefits and downsides of the strategy.
2. For endoscopy centers where the prevalence of asymptomatic SARS-CoV-2 infection is low (<0.5%), AGA suggests against implementing a pretesting strategy.
3. For a small number of endoscopy centers in high-prevalence areas, AGA suggests against implementing a pretesting strategy. In “hotspots,” endoscopy may be reserved for emergency or time-sensitive procedures with use of N95/N99 respirators or powered air purifying respirators (PAPRs) for all procedures.
4. For all endoscopy centers, AGA recommends against serologic testing as part of a pretesting strategy for patients or endoscopy staff.