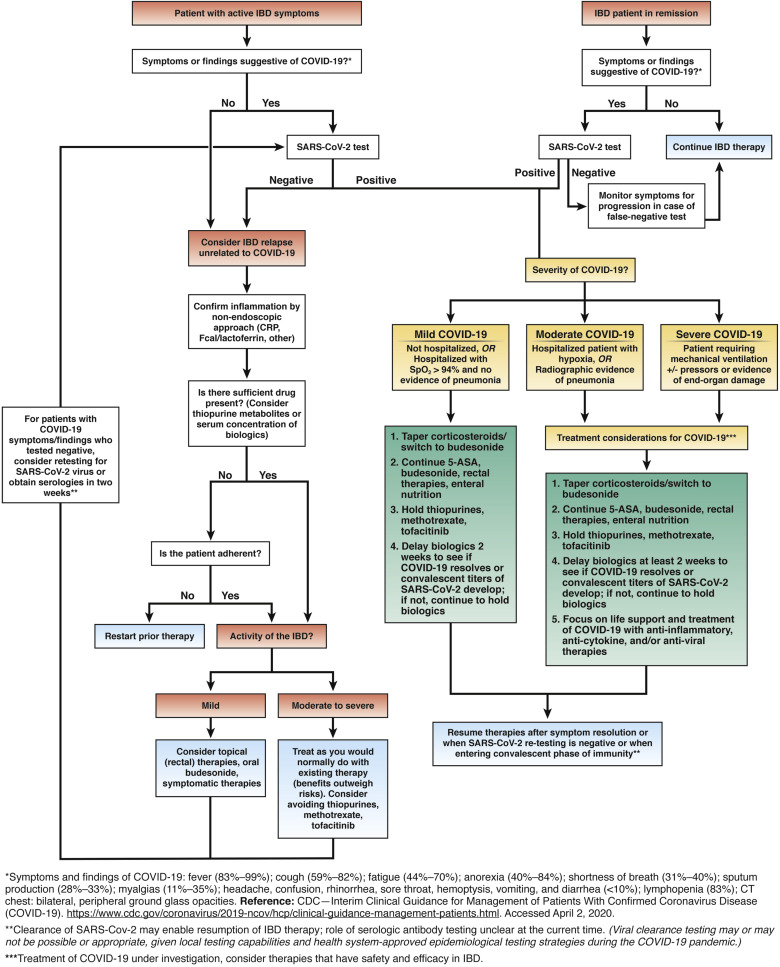
1. COVID-19 is the disease caused by the SARS-CoV-2 virus, but patients with IBD do not appear to be at a higher risk for infection with SARS-CoV-2 or development of COVID-19.
2. Patients with IBD who do not have infection with SARS-CoV-2 should not discontinue their IBD therapies and should continue infusion schedules at appropriate infusion centers.
3. Patients with IBD who have known SARS-CoV-2 but have not developed COVID-19 should hold thiopurines, methotrexate and tofacitinib. Dosing of biological therapies should be delayed for two weeks of monitoring for symptoms of COVID-19.
4. Patients with IBD who develop COVID-19 should hold thiopurines, methotrexate, tofacitinib and biological therapies during the viral illness. These can be restarted after complete symptom resolution or, if available, when follow-up viral testing is negative or serologic tests demonstrate the convalescent stage of illness.
5. The severity of the COVID-19 and the severity of the IBD should result in careful risk-benefit assessments regarding treatments for COVID-19 and escalating treatments for IBD.
6. Please submit cases of IBD and confirmed COVID-19 to the SECURE-IBD registry at covidibd.org.