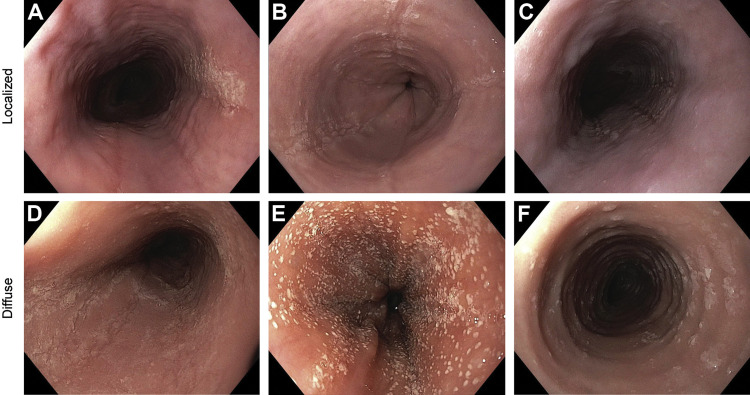
Standardized metrics to assess disease severity are used routinely to guide management choices for diseases. As eosinophilic esophagitis (EoE) — a clinicopathologic diagnosis with a pathogenesis akin to other atopic diseases, including T2 immunity, antigens and barrier disruption — becomes increasingly prevalent, the need for simple, accessible indices for severity is pressing.
In light of this, the Index of Severity for EoE (I-SEE) was created by an international team of more than 30 experts in allergy, gastroenterology and pathology. This new tool has made it possible to grade the severity of EoE using an array of clinicopathologic criteria.
Details about I-SEE
- The I-SEE has three domains: (1) symptoms and complications, (2) inflammatory features and (3) fibrostenotic.
- I-SEE can be used at initial diagnosis and then at each subsequent visit, with the recall being only between visits so that the severity can be assessed over time and ultimately (when data supports this step) treatment and monitoring adjusted based on severity.
- As the number of children and adults with EoE increases worldwide, a simple system to assess and track disease activity in a meaningful way in a clinical setting is needed.
- I-SEE is for use in adult and pediatric patients with EoE.
Validation studies
- A newly proposed severity index for EoE is associated with baseline clinical features and successful treatment response; September 2023
- The I-SEE reflects longitudinal clinicopathologic changes in children; April 2024
- Clinical and molecular correlates of the I-SEE; August 2024
- Worsening disease severity as measured by I-SEE associates with decreased treatment response to topical steroids; March 2025
- The I-SEE helps predict treatment response to dupilumab; May 2025
- Usability of a mobile point-of-care app for the I-SEE; 2025
- Assessment of real-world disease severity in patients with EoE in the U.S.; June 2025