1. In adults with active inflammatory bowel disease (IBD) treated with anti-tumor necrosis factor (TNF) agents, AGA suggests reactive therapeutic drug monitoring to guide treatment changes.
2. In adult patients with quiescent IBD treated with anti-TNF agents, AGA makes no recommendation regarding the use of routine proactive therapeutic drug monitoring.
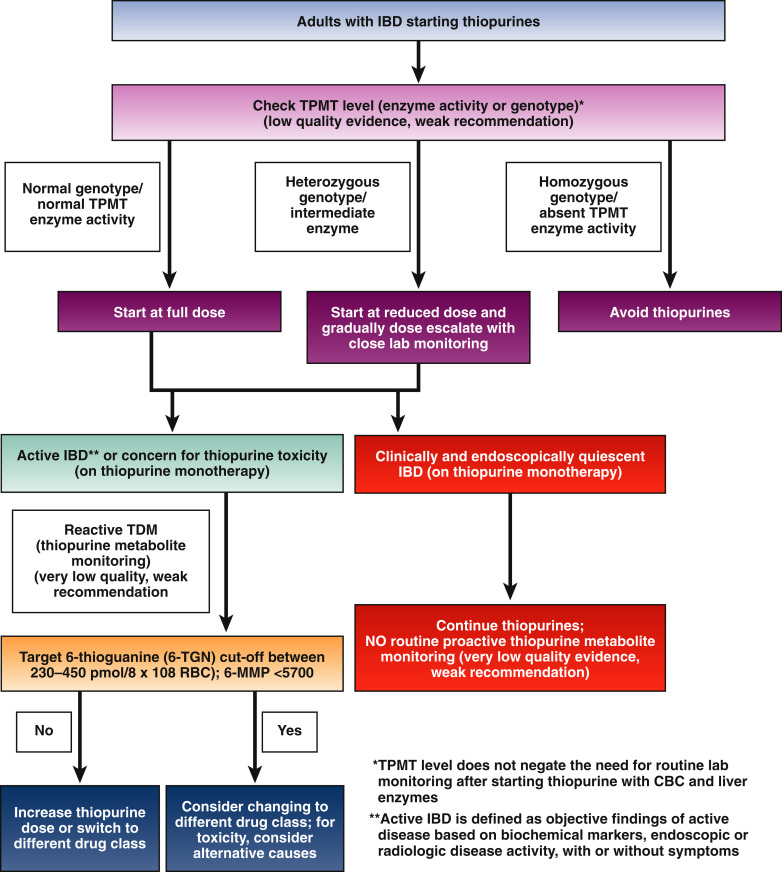
3. In adult patients with IBD being started on thiopurines, AGA suggests routine thiopurine methyltransferase (TPMT) testing (enzymatic activity or genotype) to guide thiopurine dosing.
4. In adult patients treated with thiopurines with active IBD or adverse effects thought to be due to thiopurine toxicity, AGA suggests reactive thiopurine metabolite monitoring to guide treatment changes.
5. In adult patients with quiescent IBD treated with thiopurines, AGA suggests against routine thiopurine metabolite monitoring.