AGA has published the first comprehensive evidence-based guideline on the use of fecal microbiota-based therapies for gastrointestinal disease. The guideline was published in Gastroenterology.
Key takeaways
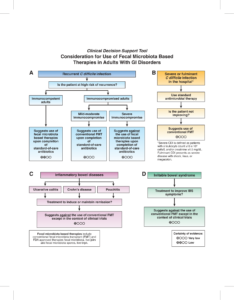
- Prevention with fecal microbiota-based therapies can be considered in patients after the second recurrence (third episode) of C. difficile infection (CDI) or in select patients at high risk for either recurrent CDI or a morbid CDI recurrence.
- In adults hospitalized with severe or fulminant CDI not responding to antimicrobial therapy, consider select use of conventional FMT as adjuvant treatment.
- Conventional fecal microbiota transplant as treatment for IBD or IBS should only be considered in the context of clinical trials.
“As a general gastroenterologist, this guideline has changed my practice. I now have a much better understanding of FMT and how to position fecal microbiota-based therapies in practice. This guideline has made me a better doctor for my C. diff patients."

This guideline covers the use of conventional fecal microbiota transplant (FMT), performed most commonly via colonoscopy, as well as recently FDA approved therapies such as fecal microbiota live-jslm (REBYOTA) delivered via enema and fecal microbiota spores live-brpk (VOWST) delivered in an oral capsule.
The guideline recommends FMT therapies only within the context of C. diff infection.
“Thanks to AGA’s new guideline, patients will suffer for shorter periods of time and be able to get back to leading happy and healthy lives."

Check out AGA’s GI Patient Center resources: