AGA Expert Exchange: Biosimilars in GI educates health care providers of the safety and efficacy of biosimilars and how to appropriately incorporate biosimilars into clinical practice.
The program kicked off with a Tweetorial, Basics in Biosimilars, covering:
- What is a biosimilar?
- How are biosimilars different than generics?
- How are biosimilars made?
- What is the FDA approval process of biosimilars?
- Prior authorization
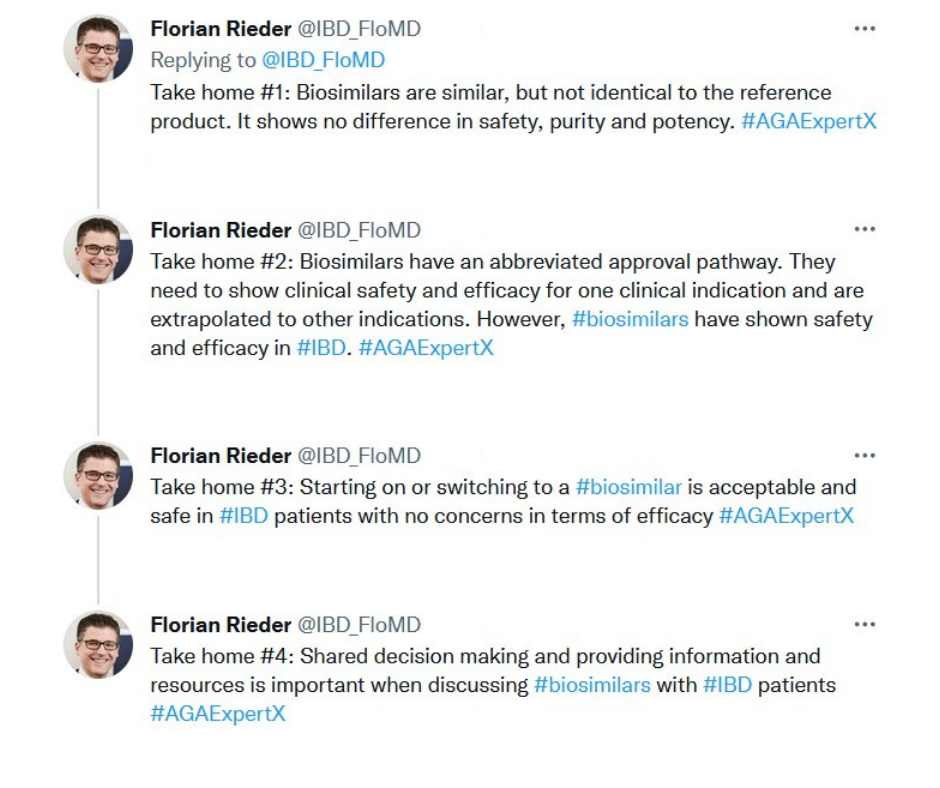
- Efficacy in IBD
- Biosimilar interchangeability
During the live Twitter chat, Biosimilars in GI, learners followed along using #AGAExpertX as our panel used cases to discuss topics like how to choose the right biosimilar for your patient and tips for shared decision-making discussions with the patient.
Drs. Kinnucan and Rieder host a Roundtable discussion with the AGA Community reviewing biosimilar cases starting Tuesday, Dec. 14.
Log in and click “follow” to get daily updates and bring your biosimilar cases and questions for our experts to address.
The final piece of the program involves a new AGA University education module coming next month, including everything covered so far.













