Through the end of the year, we’re bringing back some of the best articles from the AGA Journals blog, Digest This. Learn more about possible factors for early detection of pancreatic cancer in this article from May 2019.
Changes in blood glucose and lipid levels occur up to 18 months before a diagnosis of pancreatic cancer, researchers report in the May 2019 issue of Gastroenterology. These changes might be used in combination with other risk factors to detect pancreatic cancer at earlier stages.
One reason for the high mortality of pancreatic cancer is that it is usually not detected until an advanced stage, when it is rarely curable. Patients who present with earlier-stage disease can be treated aggressively and have better odds of long-term survival.
Patients with a family history or genetic risk factors for pancreatic cancer, and patients with pancreatic cysts, have been the primary focus of early-detection programs. However, these patients account for only 15-20 percent of cases, so other high-risk groups must be identified. Pancreatic cancer has been associated with metabolic alterations, such as hyperglycemia, which might help identify high-risk individuals for whom cancer surveillance is appropriate.
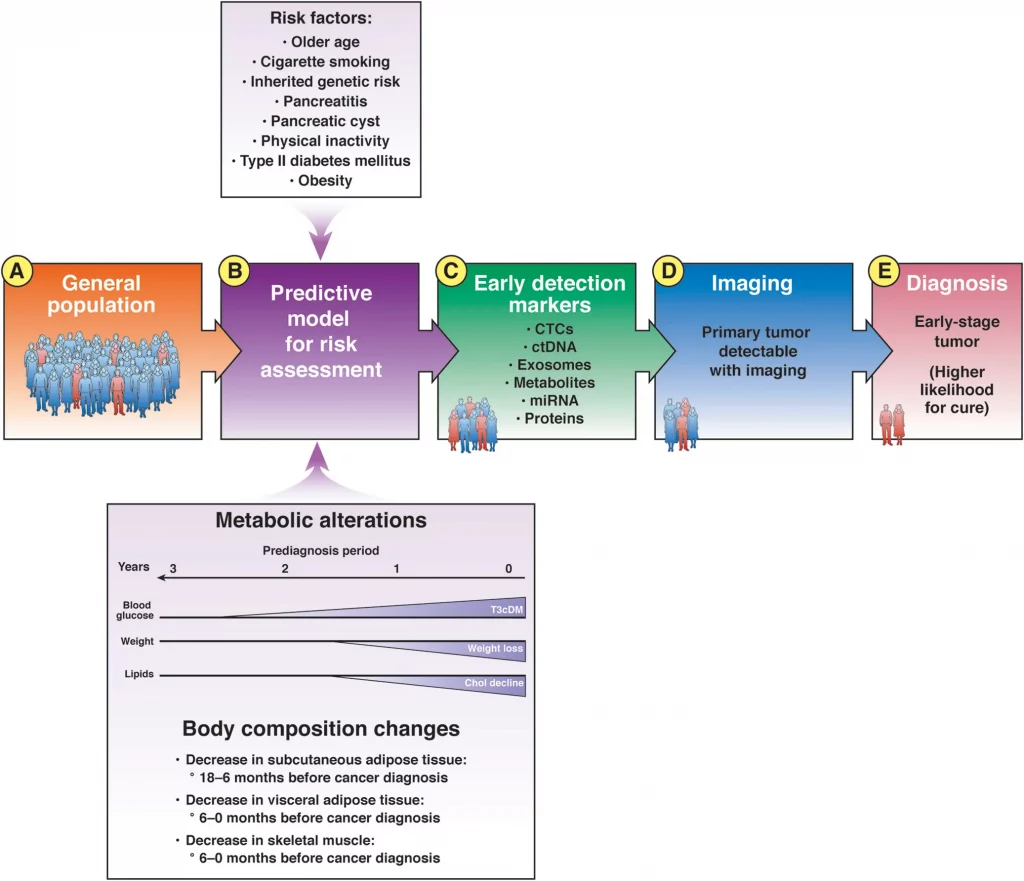
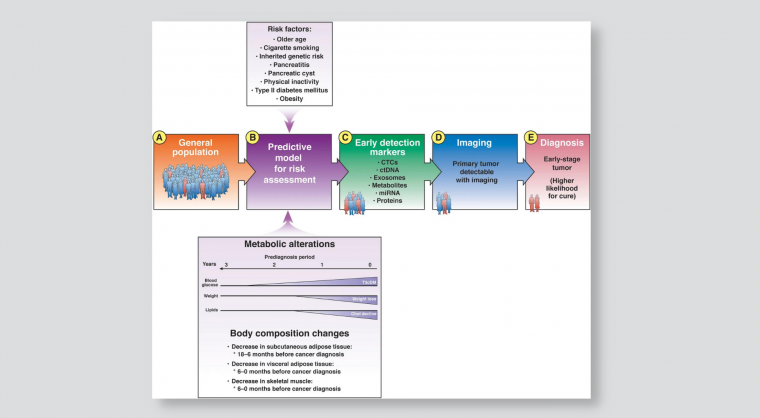
From the general population (A), models can identify individuals at increased risk for pancreatic cancer (B). The at-risk population includes many people with markers of early-stage pancreatic cancer that can be detected in blood (C); these patients can be selected for imaging analyses (D). Detection of a tumor is followed by earlier therapeutic intervention with a greater likelihood for cure (E). Chol, cholesterol; CTCs, circulating tumor cells; ctDNA, circulating tumor DNA; miRNA, microRNA; T3cDM, type IIIc diabetes mellitus.
Raghuwansh P. Sah et al collected data on 219 patients with pancreatic cancer and 657 healthy individuals (controls) from the Rochester Epidemiology Project. They analyzed differences in metabolic parameters (serum levels of lipids, triglycerides; total, low-density, and high-density cholesterol; and total body weight) and soft tissues (abdominal subcutaneous fat [SAT], adipose tissue, visceral adipose tissue [VAT], and muscle) over the 5 years before the diagnosis of pancreatic ductal adenocarcinoma (PDAC).
Sah et al observed increases in blood glucose (hyperglycemia) in the 30 to 18 months before cancer diagnosis (phase 1), without soft tissue changes.
In the 18 to 6 months before PDAC diagnosis (phase 2, pre-cachexia), patients had significant increases in hyperglycemia and decreases in serum lipids, body weight, and SAT, but not yet in VAT or muscle.
In the 6 to 0 months before PDAC diagnosis (phase 3, cachexia), a significant proportion of patients had hyperglycemia compared with controls, and patients had significant reductions in all serum lipids, SAT, VAT and muscle.
The authors studied the SAT in cell lines and mice with pancreatic cancer. Sah et al found that SAT adipocytes exposed to exosomes from plasma of patients with pancreatic cancer have lower triglycerides than adipocytes exposed to exosomes from controls. Analysis of mRNA sequences of SAT exposed to exosomes from patients with PDAC showed promotion of lipolysis, fibrosis, browning, and acute inflammation.
SAT from mice with pancreatic cancer, compared to control mice, also had signs of fibrosis, infiltration by immune cells, and reduction in adipocyte size. SAT from mice with pancreatic cancer also had increased expression of uncoupling protein 1 (UCP1), which is a mitochondrial anion carrier protein that separates oxidative phosphorylation from ATP synthesis with energy dissipated as heat. Sah observed expression of UCP1 in SAT from all 5 patients with pancreatic cancer analyzed, but only 1 of 4 controls.
Sah et al proposed that patients with pancreatic cancer have browning of SAT, based on increases in body temperature, starting 18 months before PDAC diagnosis.
Sah et al conclude that, compared with controls, patients with pancreatic cancer go through 3 distinct metabolic phases. The earliest metabolic change (phase 1) is new-onset hyperglycemia starting nearly 3 years before diagnosis. The next metabolic change (phase 2) is a decrease in lipids associated with weight loss starting 1.5 years before diagnosis. The first evidence of soft tissue change, namely SAT loss, occurs during this period. In the final phase (phase 3), starting <6 months before diagnosis, all parameters decrease (all lipids, SAT, VAT, and muscle) except fasting glucose, which continues to increase.
In an editorial that accompanies the article, Natalia Khalaf and Brian M. Wolpin state that although the findings of Sah et al provide support for the changes in metabolism before a diagnosis of pancreatic cancer, their clinical usefulness requires further investigation and could depend on the population studied. Khalaf and Wolpin state that the changes identified by Sah et al are not specific to the development of pancreatic cancer and the mean differences between cases and controls are modest.
Khalaf and Wolpin write that these factors alone are unlikely to detect early-stage pancreatic cancer with sufficient sensitivity or specificity. However, they might be included in multiparameter models and used to enrich the screening population for individuals at higher risk, while decreasing the number of false-positive results.
Multiparameter risk models have already been developed for patients with new-onset diabetes, leading to the identification of individuals with a greater than 2.5% increase in risk for pancreatic cancer in the next 3 years.

Kristine Novak, MD
Dr. Novak is a science writer and editor based in San Francisco. She has extensive experience covering gastroenterology, hepatology, immunology, oncology, clinical and biotechnology research discoveries.