1. AGA recommends that before starting any pancreatic cyst surveillance program, patients should have a clear understanding of programmatic risks and benefits.
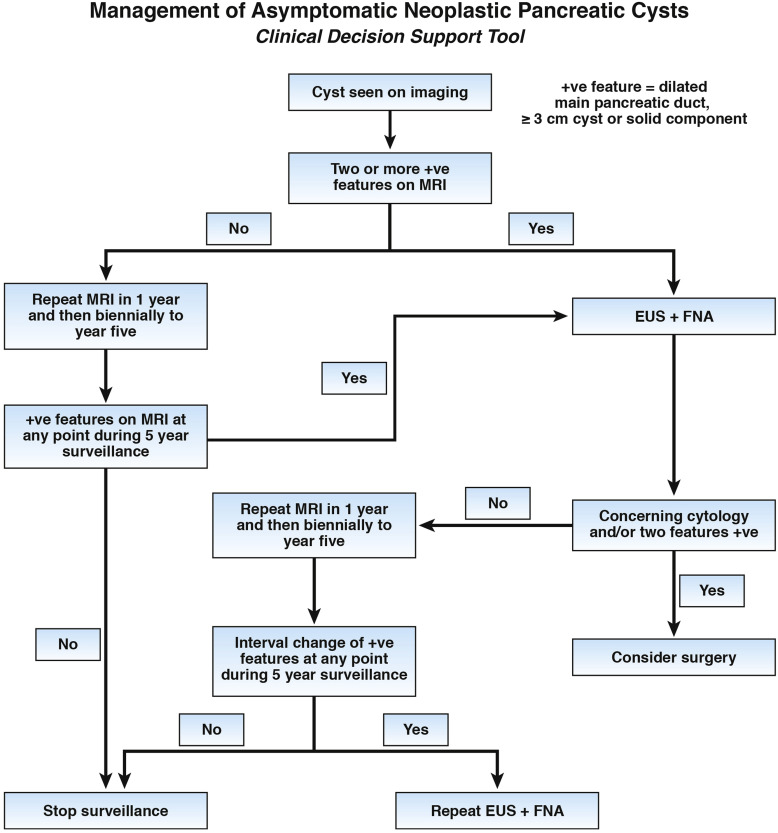
2. AGA suggests that patients with pancreatic cysts <3 cm without a solid component or a dilated pancreatic duct undergo magnetic resonance imaging (MRI) for surveillance in 1 year and then every 2 years for a total of 5 years if there is no change in size or characteristics.
3. AGA suggests that pancreatic cysts with at least two high-risk features, such as size ≥3 cm, a dilated main pancreatic duct, or the presence of an associated solid component, should be examined with endoscopic ultrasonography (EUS) – fine-needle aspiration (FNA).
4. AGA suggests that patients without concerning EUS-FNA results should undergo MRI surveillance after 1 year and then every 2 years to ensure no change in risk of malignancy.
5. AGA suggests that significant changes in the characteristics of the cyst, including the development of a solid component, increasing size of the pancreatic duct, and/or diameter ≥3 cm, are indications for EUS-FNA.
6. AGA suggests against continued surveillance of pancreatic cysts if there has been no significant change in the characteristics of the cyst after 5 years of surveillance or if the patient is no longer a surgical candidate.
7. AGA suggests that patients with both a solid component and a dilated pancreatic duct and/or concerning features on EUS and FNA should undergo surgery to reduce the risk of mortality from carcinoma.
8. AGA recommends that if surgery is considered for a pancreatic cyst, patients are referred to a center with demonstrated expertise in pancreatic surgery.
9. AGA suggests that patients with invasive cancer or dysplasia in a cyst that has been surgically resected should undergo MRI surveillance of any remaining pancreas every 2 years.
10. AGA suggests against routine surveillance of pancreatic cysts without high-grade dysplasia or malignancy at surgical resection.