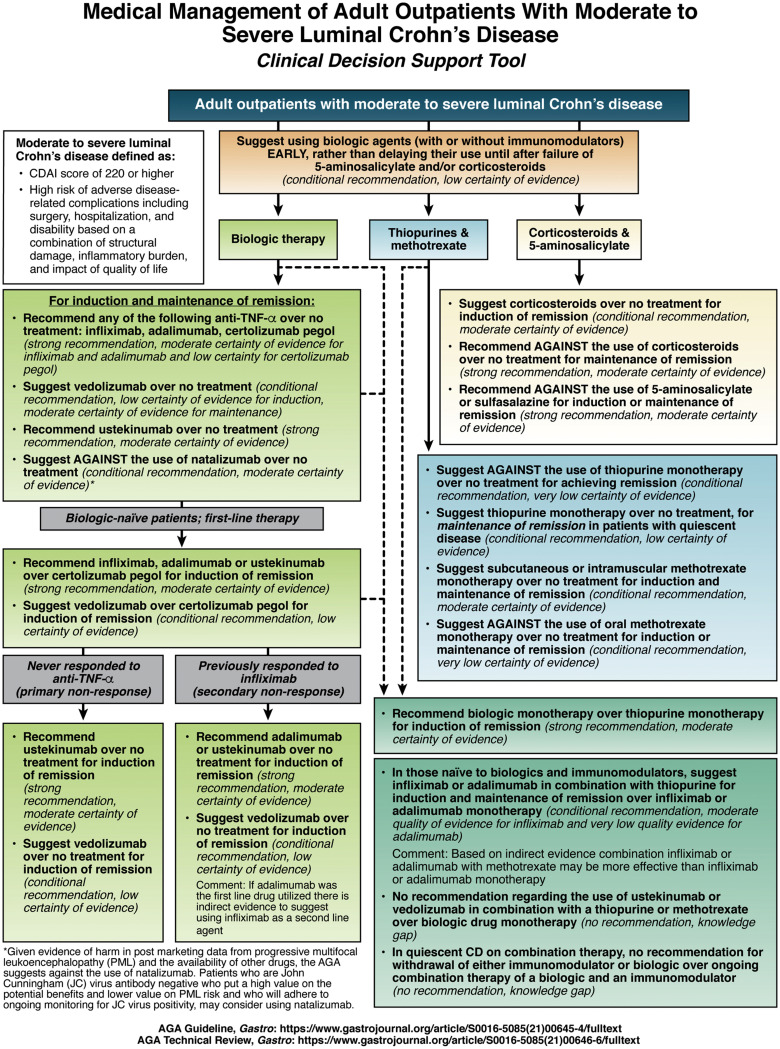
1A. In adult outpatients with moderate to severe Crohn’s disease, AGA recommends the use of anti-TNFα over no treatment for induction and maintenance of remission.
1B. In adult outpatients with moderate to severe Crohn’s disease, AGA suggests the use of vedolizumab over no treatment for the induction and maintenance of remission.
1C. In adult outpatients with moderate to severe Crohn’s disease, AGA recommends the use of ustekinumab over no treatment for the induction and maintenance of remission.
1D. In adult outpatients with moderate to severe Crohn’s disease, AGA suggests against the use of natalizumab over no treatment for the induction and maintenance of remission.
2A. In adult outpatients with moderate to severe Crohn’s disease who are naïve to biologic drugs, AGA recommends the use of infliximab, adalimumab or ustekinumab over certolizumab pegol for the induction of remission and suggests the use of vedolizumab over certolizumab pegol for the induction of remission.
2B. In adult outpatients with moderate to severe Crohn’s disease who never responded to anti-TNFα (primary nonresponse), AGA recommends the use of ustekinumab and suggests the use of vedolizumab over no treatment for the induction of remission.
2C. In adult outpatients with moderate to severe Crohn’s disease who previously responded to infliximab (secondary nonresponse), AGA recommends the use of adalimumab or ustekinumab and suggests the use of vedolizumab over no treatment for the induction of remission.
3A. In adult outpatients with moderate to severe Crohn’s disease, AGA suggests against the use of thiopurines over no treatment for achieving remission.
3B. In adult outpatients with quiescent moderate to severe Crohn’s disease (or patients in corticosteroid-induced remission), AGA suggests the use of thiopurines over no treatment for the maintenance of remission.
3C. In adult outpatients with moderate to severe Crohn’s disease, AGA suggests the use of subcutaneous or intramuscular methotrexate monotherapy over no treatment for the induction and maintenance of remission.
3D. In adult outpatients with moderate to severe Crohn’s disease, AGA suggests against the use of oral methotrexate monotherapy over no treatment for the induction and maintenance of remission.
4. In adult outpatients with moderate to severe Crohn’s disease, AGA recommends the use of biologic drug monotherapy over thiopurine monotherapy for the induction of remission.
5A. In adult outpatients with moderate to severe Crohn’s disease who are naïve to biologics and immunomodulators, AGA suggests the use of infliximab in combination with thiopurines for the induction and maintenance of remission over infliximab monotherapy.
5B. In adult outpatients with moderate to severe Crohn’s disease who are naïve to biologics and immunomodulators, AGA suggests the use of adalimumab in combination with thiopurines for the induction and maintenance of remission over adalimumab monotherapy.
5C. In adult outpatients with moderate to severe Crohn’s disease, AGA makes no recommendation regarding the use of, ustekinumab or vedolizumab in combination with thiopurines or methotrexate over biologic drug monotherapy for the induction and maintenance of remission.
6. In adult outpatients with quiescent Crohn’s disease on combination therapy, AGA makes no recommendation for withdrawal of either the immunomodulator or the biologic over ongoing combination therapy of a biologic and an immunomodulator.
7. In adult outpatients with moderate to severe Crohn’s disease, AGA suggests early introduction with a biologic with or without an immunomodulator rather than delaying their use until after failure of 5-aminosalicylates and/or corticosteroids.
8A. In adult outpatients with moderate to severe Crohn’s disease, AGA suggests the use of corticosteroids over no treatment for induction of remission.
8B. In adult outpatients with moderate to severe Crohn’s disease, AGA recommends against the use of corticosteroids over no treatment for maintenance of remission.
9. In adult outpatients with moderate to severe Crohn’s disease, AGA recommends against the use of 5-aminosalicylates or sulfasalazine over no treatment for the induction or maintenance of remission.
10A. In adult outpatients with Crohn’s disease and active perianal fistula, AGA recommends the use of infliximab over no treatment for the induction and maintenance of fistula remission.
10B. In adult outpatients with Crohn’s disease and active perianal fistula, AGA suggests the use of adalimumab, ustekinumab or vedolizumab over no treatment for the induction or maintenance of fistula remission.
10C. In adult outpatients with Crohn’s disease and active perianal fistula without perianal abscess, AGA suggests against the use of antibiotics alone over no treatment for the induction of fistula remission.
11. In adult outpatients with Crohn’s disease and active perianal fistula without perianal abscess, AGA recommends the use of biologic agents in combination with an antibiotic over a biologic drug alone for the induction of fistula remission.