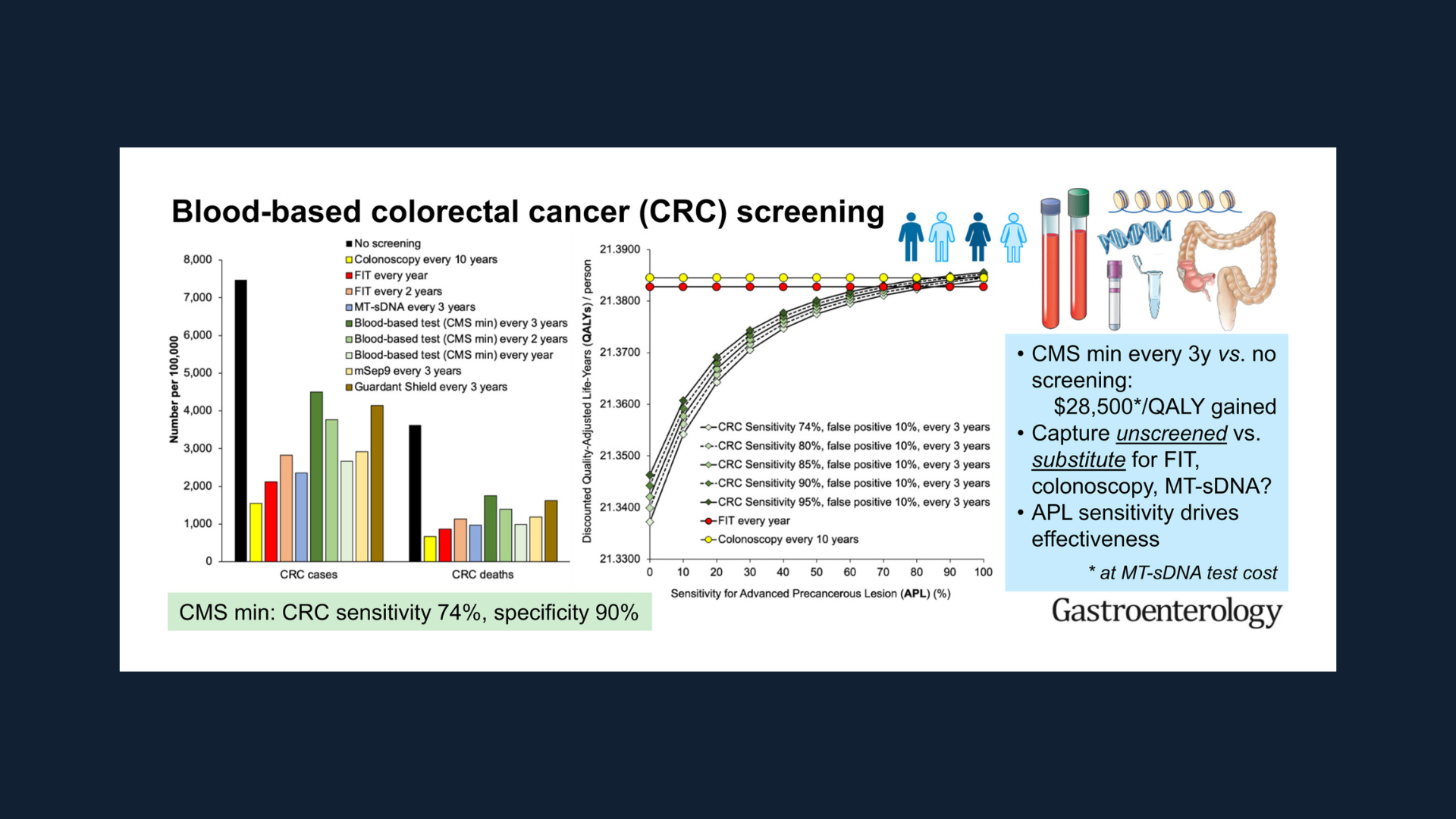
- A blood test for CRC that meets minimal CMS criteria for sensitivity and performed every three years would likely result in better outcomes than no screening.
- A blood test for CRC offers a simple process that could encourage more people to participate in screening. Patients who may have declined colonoscopy should understand the need for a colonoscopy if findings are abnormal.
- Because blood tests for CRC are predicted to be less effective and more costly than currently established screening programs, they cannot be recommended to replace established effective screening methods.
- Although blood tests would improve outcomes in currently unscreened people, substituting blood tests for a currently effective test would worsen patient outcomes and increase cost.
- Potential benchmarks that industry might use to assess an effective blood test for CRC going forward would be sensitivity for stage I-III CRC of >90%, with sensitivity for advanced adenomas of > 40-50%.
This commentary was created by an expert panel convened in September 2023 for the AGA CRC Workshop and considered modeling performed by two independent groups, including a team from the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer consortium and a team from Stanford University. These modeling studies are published in Gastroenterology:
Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening With a Blood Test That Meets the Centers for Medicare & Medicaid Services Coverage Decision
https://www.gastrojournal.org/article/S0016-5085(24)00174-4/fulltext
DOI: 10.1053/j.gastro.2024.02.012
Comparative Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening With Blood-Based Biomarkers (Liquid Biopsy) vs Fecal Tests or Colonoscopy
https://www.gastrojournal.org/article/S0016-5085(24)00293-2/fulltext
DOI: 10.1053/j.gastro.2024.03.011
AGA CRC Workshop panelists:
- David Lieberman, Oregon Health and Science University
- Aasma Shaukat, NYU Grossman School of Medicine
- Folasade P. May, University of California Los Angeles
- John M. Carethers, University of California San Diego
- Iris Lansdorp-Vogelaar, Erasmus MC, University Medical Center, Rotterdam
- Uri Ladabaum, Stanford University School of Medicine
- Timothy R. Church, University of Minnesota
- Anjelica Davis, Fight Colorectal Cancer
- Chyke A. Doubeni, MD, MPH, The Ohio State University
- John M. Inadomi, University of Utah Health
- Pedro Nascimento de Lima, RAND Corporation
- Rosita van den Puttelaar, Erasmus MC, University Medical Center, Rotterdam