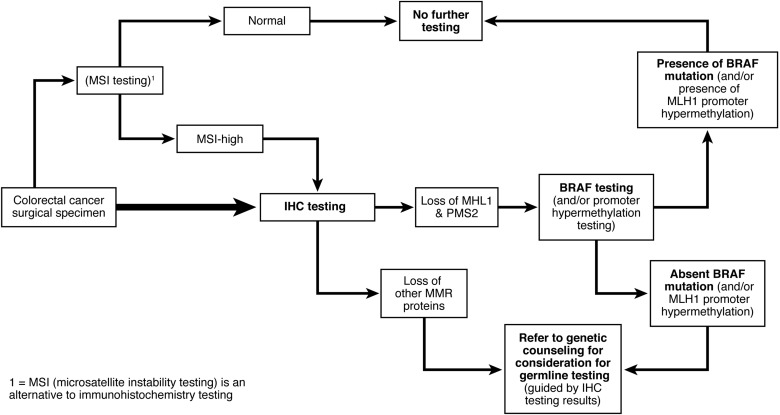
1. Testing for mismatch repair (MMR) deficiency of newly diagnosed colorectal cancer (CRC) should be performed. This can be done for all CRCs, or CRC diagnosed at age 70 years or younger, and in individuals older than 70 years who have a family history concerning for Lynch syndrome. Analysis can be done by immunohistochemistry (IHC) testing for the MLH1/MSH2/MSH6/PMS2 proteins and/or testing for microsatellite instability (MSI). Tumors that demonstrate loss of MLH1 should undergo BRAF testing or analysis of MLH1 promoter hypermethylation.
2. Individuals who have a personal history of a tumor showing evidence of MMR deficiency (without evidence of MLH1 promoter methylation); uterine cancer diagnosed at younger than age 50 years; a known family MMR gene mutation; fulfill Amsterdam criteria or revised Bethesda guidelines; and/or have a personal risk of ≥5% chance of Lynch syndrome based on prediction models should undergo genetic evaluation for Lynch syndrome.
3. Screening for CRC by colonoscopy is recommended in persons at risk (first-degree relatives of those affected) or affected with Lynch syndrome every 1 to 2 years, beginning between ages 20-25 years or 2-5 years before the youngest age of diagnosis of CRC in the family if diagnosed before age 25 years.
4. Screening for endometrial cancer (EC) should be offered to women at risk for or affected with Lynch syndrome by pelvic examination and endometrial sampling annually starting at age 30-35 years.
5. Hysterectomy and bilateral salpingo-oophorectomy should be recommended to women with Lynch syndrome who have finished childbearing or at age 40 years.
6. Screening for gastric cancer should be considered in persons at risk for or affected with Lynch syndrome by esophagogastroduodenoscopy (EGD) with gastric biopsy of the antrum at age 30-35 years with treatment of H. pylori infection when found. Subsequent, surveillance every 2-3 years can be considered based on individual patient risk factors.
7. Routine screening of the small intestine is not recommended.
8. Screening for cancer of the urinary tract should be considered for persons at risk for or affected with Lynch syndrome, with urinalysis annually starting at age 30-35 years.
9. Routine screening of the pancreas is not recommended.
10. Routine screening of the prostate and breast cancer is not recommended beyond what is advised for the general population.
11. Colectomy with ileorectal anastomosis is the primary treatment of patients affected with Lynch syndrome with colon cancer or colon neoplasia not removable by endoscopy.
12. Growing but not conclusive evidence exists that use of aspirin is beneficial in preventing cancer in Lynch syndrome patients. Treatment of an individual patient with aspirin is a consideration after discussion of patient-specific risks, benefits and uncertainties of treatment is conducted.