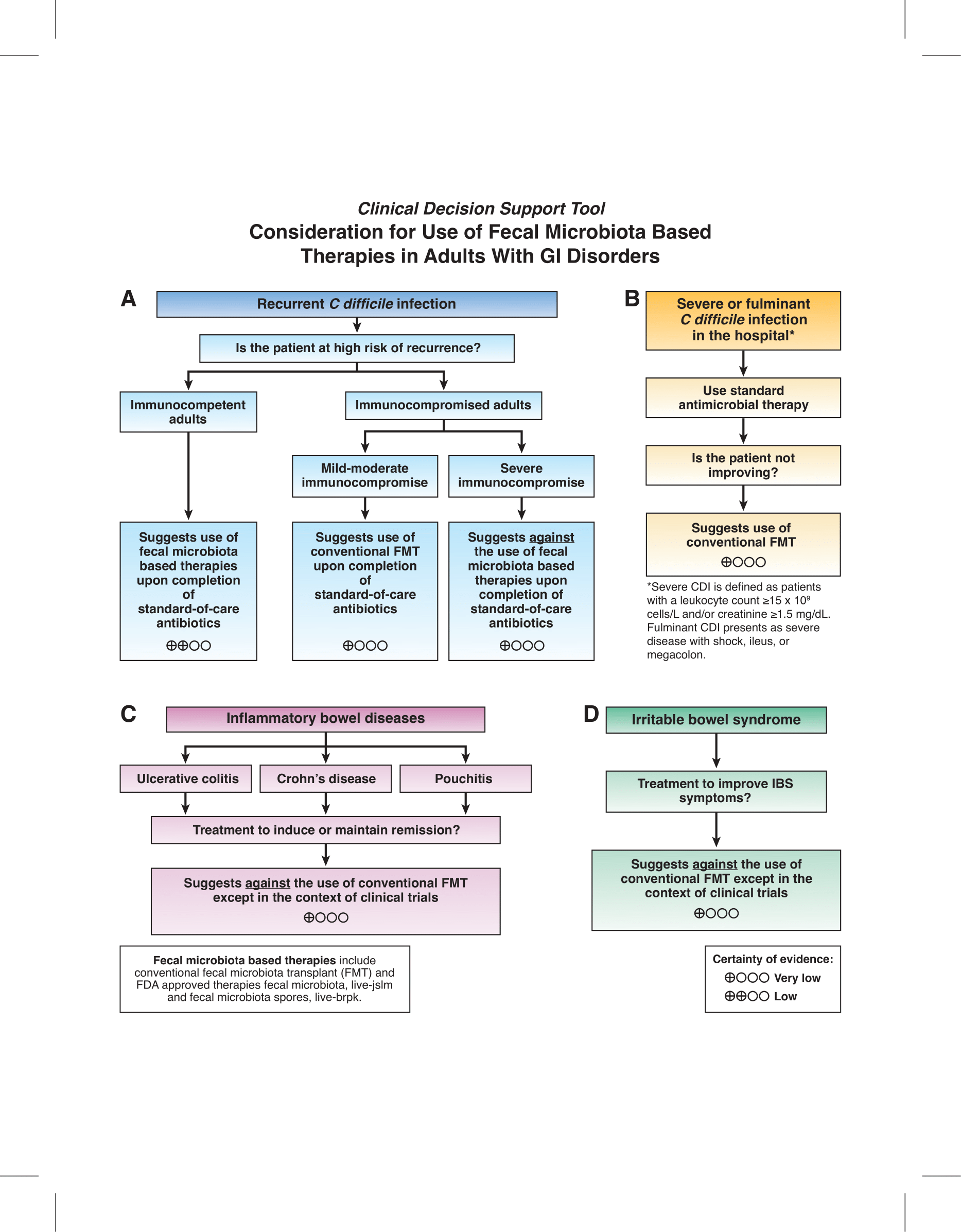
1. In immunocompetent adults with recurrent C. difficile infection, the AGA suggests the use of fecal microbiota-based therapies upon completion of standard-of-care antibiotics over no fecal microbiota-based therapies (conditional recommendation, low certainty evidence).
2. In mildly or moderately immunocompromised adults with recurrent C. difficile infection, the AGA suggests the use of conventional FMT upon completion of standard-of-care antibiotics over no FMT (conditional recommendation, very low certainty evidence). In severely immunocompromised adults with recurrent C. difficile infection, the AGA suggests against the use of fecal microbiota-based therapies upon completion of standard-of-care antibiotics over no fecal microbiota-based therapies (conditional recommendation, very low certainty evidence).
3. In adults hospitalized with severe or fulminant C. difficile infection not responding to antimicrobial therapy, the AGA suggests the use of conventional FMT over no FMT (conditional recommendation, very low certainty evidence).
4. In adults with ulcerative colitis, the AGA suggests against the use of conventional FMT except in the context of clinical trials (conditional recommendation, very low certainty of evidence).
5. In adults with Crohn’s disease, the AGA suggests against the use of conventional FMT except in the context of a clinical trial (conditional recommendation, very low certainty of evidence).
6. In adults with pouchitis, the AGA suggests against the use of conventional FMT except in the context of clinical trials (conditional recommendation, very low certainty of evidence).
7. In adults with irritable bowel syndrome, the AGA suggests against the use of conventional FMT except in the context of clinical trials (conditional recommendation, very low certainty of evidence).