1. In patients with UC in symptomatic remission, AGA suggests a monitoring strategy that combines biomarkers and symptoms, rather than symptoms alone.
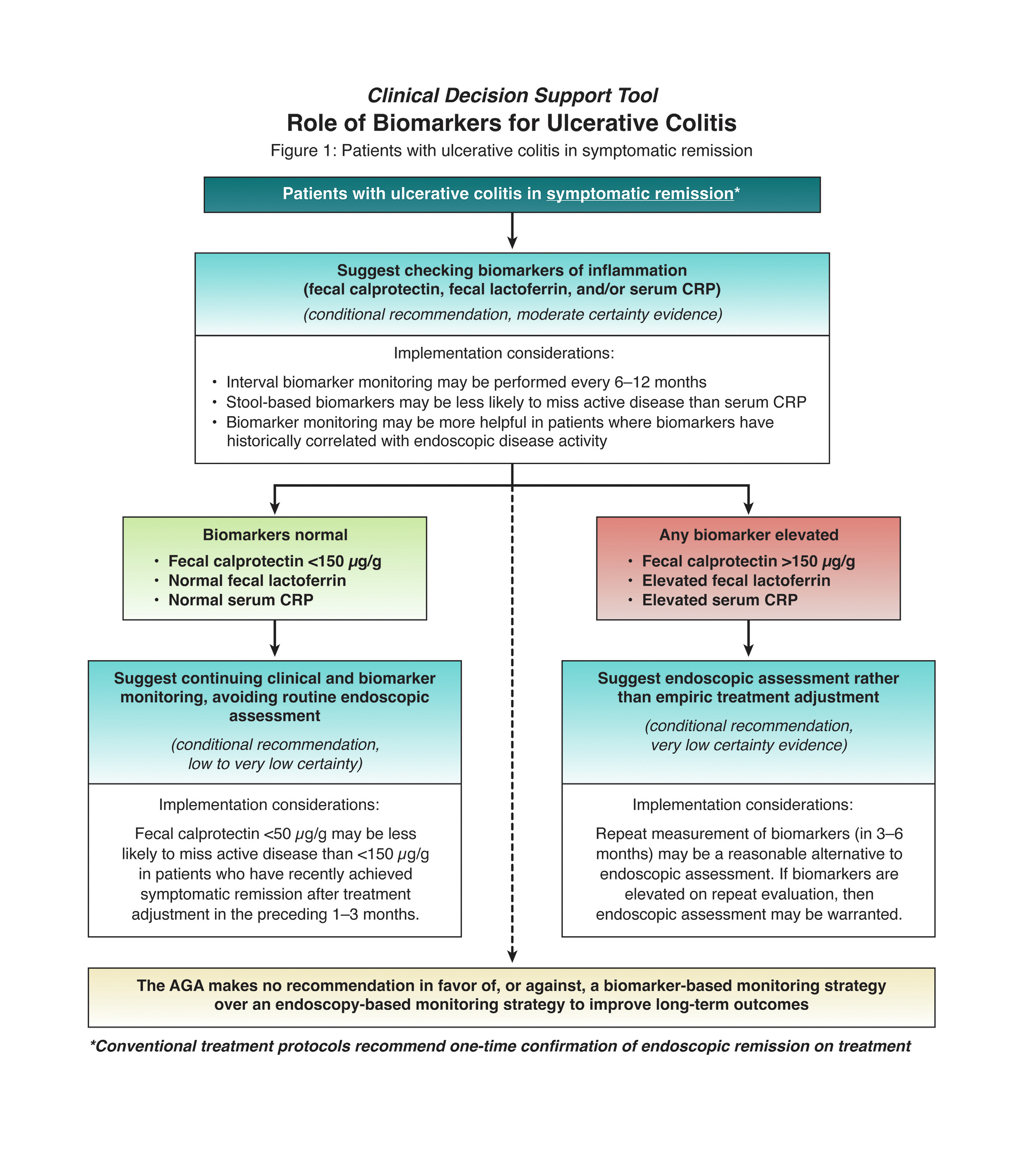
2. In patients with UC in symptomatic remission, AGA suggests using fecal calprotectin <150 μg/g, normal fecal lactoferrin, or normal C-reactive protein (CRP) to rule out active inflammation and avoid routine endoscopic assessment of disease activity.
3. In patients with UC in symptomatic remission but elevated stool or serum markers of inflammation (fecal calprotectin >150 μg/g, elevated fecal lactoferrin, elevated CRP), AGA suggests endoscopic assessment of disease activity rather than empiric treatment adjustment.
4. In patients with UC with mild symptoms, with normal stool or serum markers of inflammation (fecal calprotectin <150 μg/g, normal fecal lactoferrin, normal CRP), AGA suggests endoscopic assessment of disease activity rather than empiric treatment adjustment.
5. In patients with symptomatically active UC, AGA suggests an evaluation strategy that combines biomarkers and symptoms, rather than symptoms alone, to inform treatment adjustments.
6. In patients with UC with moderate to severe symptoms suggestive of flare, AGA suggests using fecal calprotectin >150 μg/g, elevated fecal lactoferrin, or elevated CRP to rule in active inflammation and inform treatment adjustment and avoid routine endoscopic assessment solely for establishing presence of active disease.
7. In patients with UC with mild symptoms, with elevated stool or serum markers of inflammation (fecal calprotectin >150 μg/g, elevated fecal lactoferrin, or elevated CRP), AGA suggests endoscopic assessment of disease activity rather than empiric treatment adjustment.
8. In patients with UC, AGA makes no recommendation in favor of, or against, a biomarker-based monitoring strategy over an endoscopy-based monitoring strategy to improve long-term outcomes.