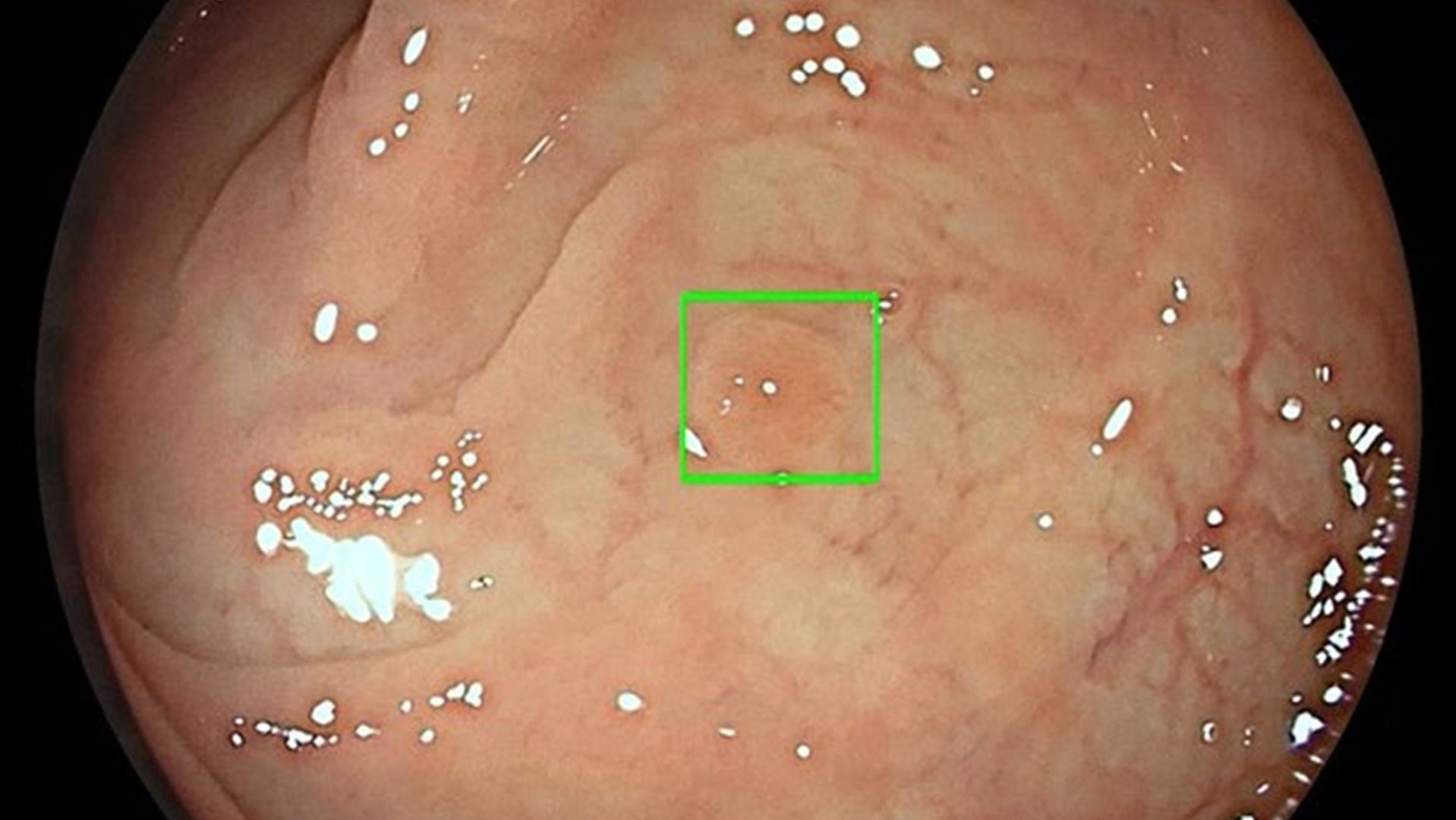
This week, the U.S. FDA authorized marketing of GI Genius™, the first device that uses artificial intelligence (AI) based on machine learning to assist clinicians in detecting lesions in the colon in real time during a colonoscopy.
Early data suggests that the use of AI in colonoscopy can improve adenoma detection rates and may ultimately reduce the risk of missed colorectal cancer. This news represents a great step forward for innovation in gastrointestinal endoscopy that will significantly impact patient outcomes.

Key points for GIs
- The device comprises hardware and software that uses AI to identify and highlight, in real time, regions of interest where further assessment may be needed. It is up you, the clinician, to decide whether the identified region actually contains a suspected lesion, and how the lesion should be managed and processed per standard clinical practice and guidelines.
- The software is compatible with most standard video endoscopy systems.
- FDA assessed the safety and effectiveness of the GI Genius through a multicenter, prospective, randomized, controlled study in Italy with 700 subjects 40-80 years old who were undergoing a colonoscopy for colorectal cancer screening, surveillance, positive results from a previous FIT test, or symptoms of possible colorectal cancer. The results were published last year in Gastroenterology.
- While use of this device led to more biopsies being performed, there were no adverse events reported with the additional biopsies, such as perforations, infections or bleeding. However, there was a slight increase in the number of lesions biopsied that were not adenomas.
More information is available in the FDA announcement.
Dr. Komanduri is professor of medicine and surgery at Northwestern University, Chicago.