Key messages
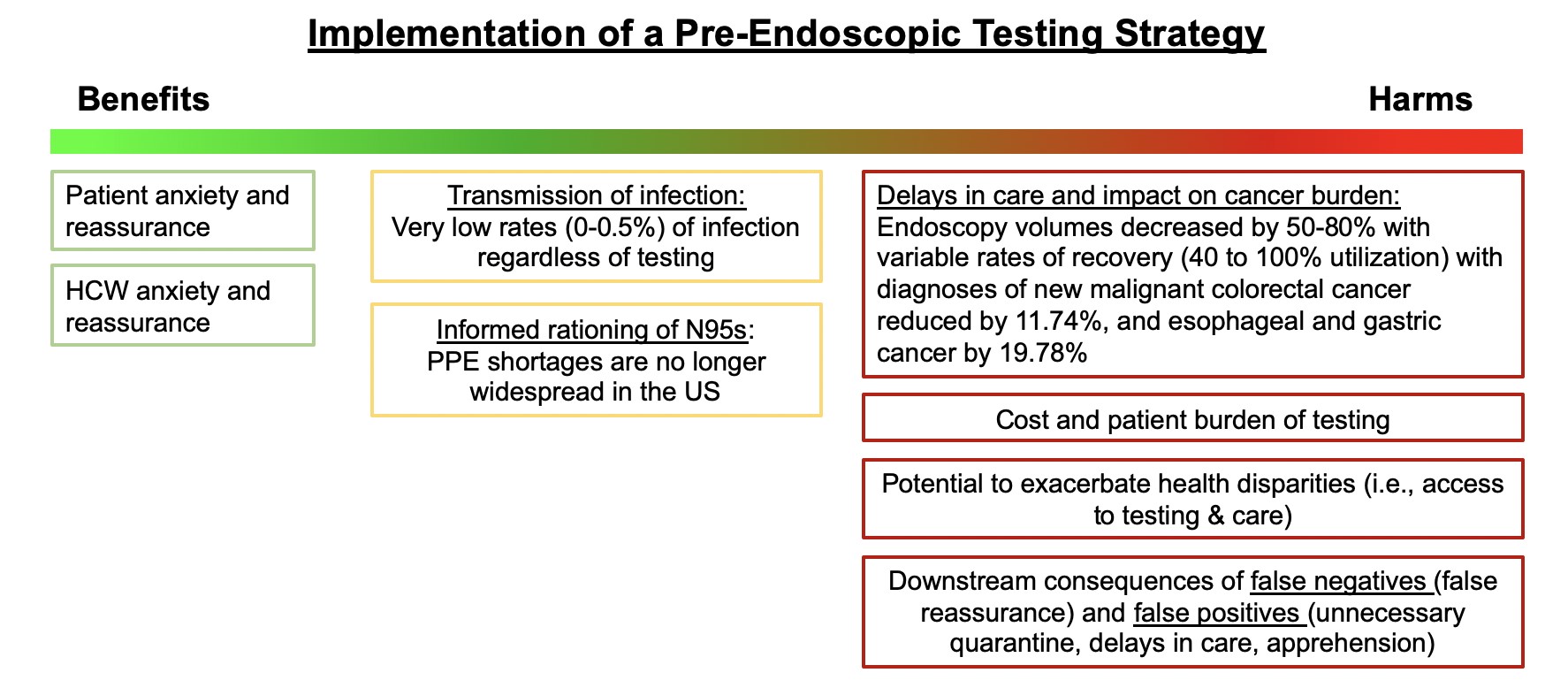
- AGA suggests against re-instituting routine pre-procedure testing prior to elective endoscopy. The downsides (delays in patient care, burden, inaccurate results) outweigh potential benefits. Infection and transmission of SARS-CoV2 from asymptomatic individuals is rare especially among vaccinated health care workers using personal protective equipment (PPE), even with the emergence of the Delta variant.
- If PPE is available, AGA recommends using N95 for upper endoscopy and suggests using N95 or surgical masks for lower endoscopy (acknowledging that upper endoscopy is more aerosolizing than lower endoscopy) and continuation of elective and non-elective endoscopy.
- Based on local prevalence rates, PPE and test availability, in intermediate and high prevalence settings, pre-procedure testing may be used to inform PPE decisions (N95 versus surgical mask). Additional benefits to testing are small and include deferring elective endoscopy in individuals testing positive and reducing anxiety among staff and patients.
As COVID-19 cases rise in the U.S. due to the Delta variant, there is renewed concern about infection and transmission of SARS-CoV2 during endoscopy. In May 2021, AGA released updated recommendations on pre-procedure testing post-vaccination in the setting of ongoing population-wide vaccination programs for the prevention of COVID-19 related morbidity. In vaccinated individuals, breakthrough infections occurred very infrequently. Weighing the evidence demonstrating extremely low rates of rates of infection and transmission with vaccination and PPE, and considering the downsides of routine testing (burden, cost, false test results, increased disparities), AGA made a conditional recommendation against routine pre-procedure testing for elective cases. The highly contagious Delta variant has now emerged as the predominant SARS-CoV2 virus in the U.S. and some data suggests that it may cause more severe illness than previous strains. While more breakthrough infections may develop in fully vaccinated individuals, the greatest risk of infection, transmission and hospitalizations is among those who are unvaccinated.
Recommendation
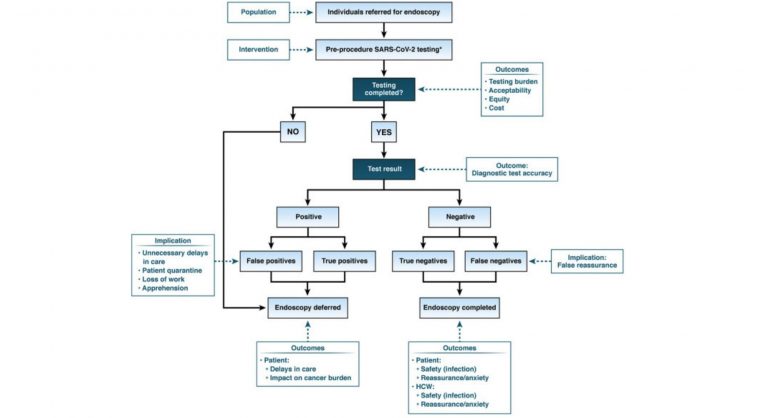
AGA suggests against routine preprocedure testing for SARS-CoV2 in patients undergoing upper or lower endoscopy, irrespective of vaccination status of patients.
Updated recommendations: Fall 2021
Should pre-procedure testing be re-instituted for all elective endoscopy cases?
AGA suggests against re-instituting routine pre-procedure testing prior to elective endoscopy. The downsides (delays in patient care, burden, inaccurate results) outweigh any small potential benefits. Infection and transmission of SARS-CoV2 from asymptomatic individuals is rare especially among vaccinated healthcare workers, even with the emergence of the Delta variant.
If PPE is available, AGA recommends using N95 for upper endoscopy and suggests using N95 or surgical masks for lower endoscopy (acknowledging that upper endoscopy is more aerosolizing than lower endoscopy) and continuation of elective and non-elective endoscopy.
Any consideration to re-institute or continue pre-procedure testing for SARS-CoV2 should be based on local prevalence of SARS-CoV2 within one’s community or local geographic context because of the varying rates of COVID resurgence. If routine pre-procedural testing is being considered;
- AGA recommends that individuals follow the guidance provided in the following guideline document, released on August 2020: AGA Institute Rapid Review and Recommendations on the Role of Pre-Procedure SARS-CoV2 Testing and Endoscopy
- AGA suggests using standard nucleic acid testing (laboratory-based NAAT or rapid RT-PCR) rather than a rapid isothermal test or antigen tests. Rapid RT-PCR tests that can be easily performed on the day of endoscopy (results available within 1 hour) are preferable as they pose less burden to patients. In the pre-procedure setting, the utility of rapid isothermal tests or antigen tests is limited due to concerns regarding accuracy. There is no role for antibody tests for pre-procedure testing.
What can endoscopy units do to ensure safety during endoscopy for patients and health care workers?
Rates of COVID surges in the U.S. are inversely correlated with prevalence of vaccination, with surges occurring in communities with lowest vaccination rates. With formal FDA approval of the Pfizer-BioNTech vaccine, health systems and societies are endorsing vaccine mandates for all health care workers. AGA supports this mandate. Improving vaccination rates will decrease rates of COVID-19 infection and transmission among staff and patients. AGA also encourages all endoscopy units to symptom screen, endorse indoor masks, maintain physical distancing and limiting the number of individuals in clinical settings.
——————-
References
- Center for Disease Control and Prevention. Updated August 19, 2021
- AGA Rapid Review and Guideline for SARS-CoV2 Testing and Endoscopy Post-Vaccination: 2021 Update. Sultan S, Siddique SM, Singh S, Altayar O, Caliendo AM, Davitkov P, Feuerstein JD, Kaul V, Lim JK, Mustafa RA, Falck-Ytter Y, Inadomi JM; American Gastroenterological Association. Gastroenterology. 2021 Sep;161(3):1011-1029.e11. doi: 10.1053/j.gastro.2021.05.039.
- AGA Institute Rapid Review and Recommendations on the Role of Pre-Procedure SARS-CoV-2 Testing and Endoscopy. Sultan S, Siddique SM, Altayar O, Caliendo AM, Davitkov P, Feuerstein JD, Francis D, Inadomi JM, Lim JK, Falck-Ytter Y, Mustafa RA; American Gastroenterological Association. Electronic address: [email protected]. 2020 Nov;159(5):1935-1948.e5. doi: 10.1053/j.gastro.2020.07.043.